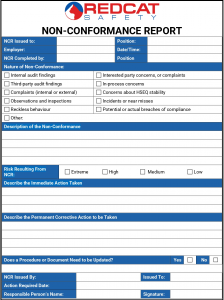
What are Non-Conformities, Corrective and Preventative Actions?
In general terms:
- A nonconformity is the non-fulfillment of a requirement, where a requirement is a need or expectation that is stated, generally implied or obligatory.
- A corrective action is an action to eliminate a detected nonconformity and to prevent a recurrence.
- A preventative action is an action to eliminate the cause of a potential nonconformity or other potential undesirable situation.
A nonconformity means there has been a deviation from a requirement. A corrective action is a specific action that needs to be taken to eliminate the root cause of that nonconformity or other undesirable situation.
Nonconformities can occur in any process, product, or service and when they do occur, they should be corrected as soon as possible to prevent them from happening again.
How to Manage a Nonconformity
When a nonconformity is identified, it is important to take quick and effective action to maintain compliance with requirements. Here are a few key steps to effectively manage a nonconformity.
1. Identify the Nonconformity
When a nonconformity is identified, it is important to take quick action to isolate the nonconformity. This means identifying the affected product, service or area and putting it under control so that the nonconformity does not continue or spread. This may involve stopping production, removing finished products from the affected area, or taking other measures to prevent the nonconformity from spreading.
2. Determine the Root Cause of the Nonconformity
Determining the root cause of nonconformity is critical to ensure that corrective and preventive actions are effective in preventing recurrence. To do this, we need to ask why the nonconformity occurred. This may be done using the ‘Five Whys’ method.
3. Implement Corrective and Preventive Actions to Address the Root Cause of the Nonconformity
Once corrective and/or preventative actions have been agreed upon, action plans should be developed (including the details of who is responsible) for applying the corrective or preventative action(s) and the appropriate timing for completion of such action(s). Allowances should be made for tracking the status of action items until such action is completed.
Note: the records of actions, including the assignment of responsibility and appropriate timeframes should be maintained for a nonconformity. This will allow the tracking of the action status until corrected, closed out and verified as effective.
4. Close Out the Nonconformity
A means of review and close-out should be followed to assess the status of action items. The review should be recorded against the action item for verification purposes.
A conformity report of all action items that remain outstanding after planned completion dates should be presented for discussion at management review meetings.
These reports should be retained as ‘documented information.’ The report should also be signed and dated by the person who was responsible for addressing the issue.
Contents of this Nonconformity and Corrective Action Procedure
- Approval.
- Purpose.
- Scope and Objectives.
- Terms and Definitions.
- Roles and Responsibilities.
- Procedures.
- Non-Conformities, Corrective and Preventative Action Process Overview.
- Non-Conformities, Corrective and Preventative Action Process Flowchart.
- Identifying a Non-Conformity.
- Repaired.
- Rejected or Scrapped.
- Returned to Supplier.
- Non-Conforming Services.
- Corrective and Preventative Actions.
- Corrective and Preventative Action Reviews.
- Related Procedures, Forms and Documents.
- Review Criteria.
- Record Management.
- References.
Why Choose to Buy this Nonconformity and Corrective Action Procedure
This 10-page nonconformity and corrective action procedure template can assist you to establish the required processes for identifying, documenting and analyzing non-conformities and meeting the requirements of objective evidence by applying appropriate corrective or preventative actions.
This procedure template can be applied to all non-conforming products, services, processes and any aspect of an ISO management system, inclusive of:
- Processes producing negative results and defective outputs.
- Products received from suppliers which are found to be non-conforming.
- Products or services provided from an external source that does not comply with the requirements of a purchase order and/or contract.
- Processes that may be identified as being non-conforming.
After purchasing this template you will be able to:
- Very easily edit and customize the template to create your own procedure.
- Apply your own style, format and brand to the procedure.
- Use it in any industry or sector regardless of the size or type of organization.
Availability and Use of this Nonconformity and Corrective Action Procedure
- This procedure is accessible to you right now by clicking the ‘Buy Now’ button.
- The procedure will be delivered to you in fully editable Microsoft Word format for immediate and full use in your business.
- There are no subscriptions, contracts or ongoing costs.
This Nonconformity and Corrective Action Procedure is 100% Satisfaction Guaranteed.


